Voor patiënten
Afdelingen

Poliklinieken en verpleegafdelingen
Informatie voor patiënten
Nieuws
Deelname aan wetenschappelijk onderzoek
Voor de voortgang van de medische wetenschap is deelname van patiënten aan wetenschappelijk onderzoek essentieel. In Nederland gebeurt dit onderzoek voor het merendeel binnen of onder leiding van de acht Universitaire Medisch Centra. Als patiënt in het LUMC kunt u gevraagd worden deel te nemen aan medisch-wetenschappelijk onderzoek.
U bezoekt het Hart Long Centrum in het Leids Universitair Medisch Centrum (LUMC) of op een van onze buitenklinieken voor medisch onderzoek en/of behandeling naar en van hart- en vaatziekten of longziekten. Voor uw diagnose en behandeling verzamelen wij verschillende gegevens van en over u, die worden opgeslagen in uw elektronisch patiëntendossier. Soms nemen wij ook lichaamsmateriaal af, zoals bloed of urine, wat veilig door ons wordt opgeslagen.
Wij zouden graag uw toestemming willen vragen om uw verzamelde gegevens en overgebleven lichaamsmateriaal te gebruiken voor het verbeteren van onze zorg door middel van onderzoek naar het ontstaan en de behandeling van hart-, vaat en longziekten. Hieronder leest u wat dit voor u betekent en welke beslissingen u hier zelf over kunt maken. Deze informatie gaat alleen over onderzoek met gegevens en lichaamsmateriaal die al van u verzameld zijn of verzameld gaan worden voor uw diagnose en behandeling. U hoeft zelf niets extra’s te doen. Om uw gegevens te mogen gebruiken hebben wij wel uw toestemming nodig. Onderstaande informatie geeft u inzicht in hoe u toestemming kunt geven en hoe dit geregistreerd wordt. Wij adviseren u onderstaande informatie goed door te nemen.
Indien deelname aan een onderzoek wel een actieve handeling van u vereist, zoals het deelnemen aan interviews of het invullen van vragenlijsten, zullen wij voor het desbetreffende onderzoek altijd nog apart om uw toestemming vragen. Lees hier de folder over toestemming van het gebruik medische gegevens patiënten.
Hieronder zijn veel gestelde vragen te vinden.
Patiëntfolders
Ons team
Video's
In samenwerking met andere afdelingen is het Hart Long Centrum Leiden een van de toonaangevende centra op het gebied van diagnostiek en behandeling van cardiovasculaire- en longaandoeningen. Het Hart Long Centrum Leiden bestaat uit een team enthousiaste medewerkers die allen met veel toewijding samenwerken met als doel goede, efficiënte en veilige zorg te bieden en de zorg voor patiënten met hart-, vaat- en longaandoeningen te optimaliseren.
Onze onderzoekers zijn dagelijks betrokken bij de ontwikkeling van nieuwe methoden voor diagnostiek en behandeling van hart-, vaat- en longziekten. Uitvoering en vernieuwing van bestaande richtlijnen als ook het verkrijgen van nieuwe inzichten hebben wij tot doel. Daarnaast staat bij ons maatschappelijke betrokkenheid hoog in het vaandel. Zo bieden wij bijvoorbeeld maatschappelijke stages aan middelbare scholieren en werken we als afdeling mee aan symposia en congressen.
Kwaliteitsbeleid
Wij werken met de PDCA (Plan Do Check Act) cyclus. Deze methode maakt het mogelijk om continu verbeteringen en eventuele vernieuwingen gecontroleerd toe te passen, te evalueren en eventueel verder te verbeteren.
Hieronder vindt u een greep uit de activiteiten van ons kwaliteitsbeleid.
Kwaliteitsteam
De Decentrale Meldingscommissie (DMC) bespreekt en analyseert de intern binnen gekomen meldingen en zet waar nodig verbeterplannen uit.
De DMC bestaat uit medewerkers uit verschillende disciplines: medisch specialisten, een klinisch fysicus, teamleiders, een kwaliteitsfunctionaris, physician assistent, verpleegkundigen en promovendi.
Naast het bespreken van de incidentmeldingen, worden complicaties, (bijna-)incidenten en fouten besproken in een maandelijkse bijeenkomst voor arts-assistenten en cardiologen. Door hier lering uit te trekken, wordt bijgedragen aan kwaliteitsverbetering.
Hieronder vindt u een overzicht van de aard van de incidenten van 2021.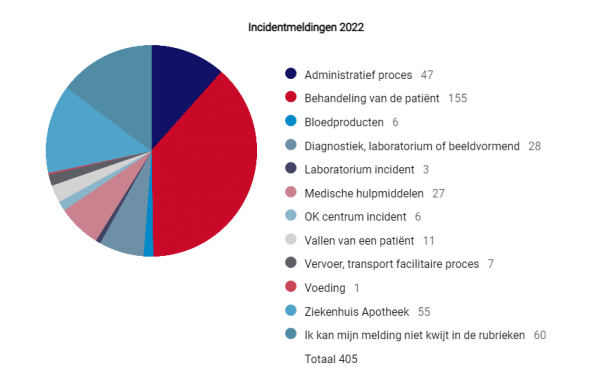
Jaarverslagen
Ieder jaar stellen wij een overzicht samen van activiteiten, resultaten en de toekomst van het Hart Long Centrum Leiden. Wij vinden het belangrijk om dit met u te delen; uiteindelijk willen we samen met u de kwaliteit van zorg verbeteren.
Hieronder kunt u de jaarverslagen van de afgelopen jaren lezen. Wij stellen het op prijs wanneer u uw vragen of opmerkingen met ons deelt. Alleen samen met u kunnen we de kwaliteit van onze zorg verbeteren. U kunt ons mailen op cardio@lumc.nl.
Maatschappelijke betrokkenheid
Het Hart Long Centrum Leiden is betrokken bij de organisatie van verschillende maatschappelijke activiteiten, zoals workshops, presentaties, evenementen en campagnes om het publiek te informeren over onze klinische en wetenschappelijke inspanningen.
Op deze manier hopen wij bij te dragen aan een beter begrip van hart-, -vaat, en longziekten en bewustwording te creëren over het belang van een gezonde levensstijl die deze ziekten kan helpen voorkomen. Bovendien moet het duidelijk worden waarom wetenschappelijk onderzoek cruciaal is voor nieuwe ontwikkelingen in cardiovasculaire en pulmonale aandoeningen en hoe wij donaties gebruiken voor ons wetenschappelijk onderzoek.
Volg ons op
EPD-Vision
Het Hart Long Centrum Leiden heeft sinds 1992 een eigen elektronisch patiëntendossier (EPD) in ontwikkeling. Al sinds 2006 werkt dankzij dit systeem (EPD-Vision) de hele afdeling (Hartziekten en Thoraxchirurgie) papierloos. Artsen, verpleegkundigen, en onderzoekers werken er dagelijks mee. Via EPD-Vison heeft de zorgverlener ook toegang tot gegevens die zich in andere systemen bevinden, zoals ECG’s, angiobeelden (cathkamers), echobeelden etcetera. Ook meetgegevens uit pacemakers en ICD’s kunnen automatisch via een koppeling met programmers en met externe databases worden ingelezen in EPD-Vision.
&width=710&height=710)
&width=710&height=710)
Contact Planningssecretariaat Hartcentrum
Op het planningssecretariaat Hartcentrum vindt de planning plaats van de onderzoeken en behandelingen op de Hartkatheterisatieafdeling en Thoraxchirurgie, zoals een dotterbehandeling, elektrofysiologie, pacemaker-/ ICD-implantatie, bypass- en klepchirurgie, longoperatie etc.
U kunt het planningssecretariaat mailen of bellen om te informeren naar de status van uw opname/behandeling.
&width=180&height=180)
&width=180&height=180)
&width=400&height=400)
&width=400&height=400)
&width=400&height=400)
&width=400&height=400)
&width=400&height=400)
&width=400&height=400)