Modulation of autoimmunity
From Autoreactive B cells to Immunological Remission
Rheumatoid Arthritis (RA), like most autoimmune diseases (AIDs), is a chronic inflammatory disease in which disease flares are common. A state of sustained, drug-free remission is rarely achieved. It is conceivable that immunological processes driving disease remain active in patients even if clinical signs and disease symptoms are well-controlled through medication. To date, the nature of such ‘immunological disease activity’ is unclear, as are the strategies required to adress it and to reach immunological remission (rather than clinical remission). Insight into these phenomena is crucial to understand disease chronicity and for strategies aiming for permanent cure. Therefore, with our team, we aim to delineate the molecular basis of (the absence of) immunological remission in RA. RA is a common and prototypic AID which has, in many ways, been at the forefront of discoveries of mechanistic insights that are applicable to AIDs on a broader scale. Hence, we learn from RA to build concepts and use our tools and knowledge to also study and translate them to other rheumatic AIDs, such as systemic sclerosis, ANCA-associated vasculitis and systemic lupus erythematosus. By doing so, we learn from their commonalities and differences.
…Rheumatoid Arthritis (RA), like most autoimmune diseases (AIDs), is a chronic inflammatory disease in which disease flares are common. A state of sustained, drug-free remission is rarely achieved. It is conceivable that immunological processes driving disease remain active in patients even if clinical signs and disease symptoms are well-controlled through medication. To date, the nature of such ‘immunological disease activity’ is unclear, as are the strategies required to adress it and to reach immunological remission (rather than clinical remission). Insight into these phenomena is crucial to understand disease chronicity and for strategies aiming for permanent cure. Therefore, with our team, we aim to delineate the molecular basis of (the absence of) immunological remission in RA. RA is a common and prototypic AID which has, in many ways, been at the forefront of discoveries of mechanistic insights that are applicable to AIDs on a broader scale. Hence, we learn from RA to build concepts and use our tools and knowledge to also study and translate them to other rheumatic AIDs, such as systemic sclerosis, ANCA-associated vasculitis and systemic lupus erythematosus. By doing so, we learn from their commonalities and differences.
As is the case for other AIDs, RA is remarkably responsive to the therapeutic depletion of B cells, which highlights B cells as important drivers of disease pathogenesis. We observed and described that RA patients harbor highly disease-specific, autoreactive B cells that are persistently active at disease-onset and during established disease, while being inactive in the ‘at-risk’ phase preceding RA. From this observation, we derive the hypothesis that only ‘quiescence’ of the autoreactive B cell response reflects true immunological remission. This quiescence is required to achieve a state of drug-free sustained remission, the ultimate proxy for cure.
To test this hypothesis and to thereby address seminal questions of autoreactive B cell biology in human AIDs, we study how these cells become activated, how their phenotype and state of activation is chronically maintained over decades, and if and how the reversion to a quiescent state can be (therapeutically) achieved.
Our aim: breaking the barrier of disease chronicity to achieve immunological remission, a proxy for cure
Innovative technology applied in the context of clinical disease trajectories
We consider therapeutic restoration of stable, long-lasting immunological remission the major next challenge for RA, either at the pre-disease stage (prevention) or in the early/established phase of disease (sustained remission/cure). The pre-requisite to achieve this goal is a sensitive approach to detect and monitor the most relevant, disease-driving immunological processes in different phases of (pre-)RA. This approach should be informative of the underlying biology and identify a targetable part of the pathogenic (auto-)immune response. Autoreactive B cells have the potential to meet these requirements and to serve as biomarkers of ‘immunological imminence’ (disease onset/flares), ‘immunological disease activity’ and ‘immunological remission’ in individual patients. Therefore, we developed a stringent, multimeric, antigen-specific multicolor flow cytometry and cell sorting technique that allows us to identify and isolate autoreactive B cells as single cells from patients. We combine this approach with state-of-the art single cell technology (such as spectral flow cytometry and single cell mRNA sequencing) and a tool-box of methods to sequence B cell receptor repertoires, express monoclonal antibodies, study their (cross-)reactivities and molecular characteristics, and generate immortalized cell lines that express specific B cell receptors.
…We consider therapeutic restoration of stable, long-lasting immunological remission the major next challenge for RA, either at the pre-disease stage (prevention) or in the early/established phase of disease (sustained remission/cure). The pre-requisite to achieve this goal is a sensitive approach to detect and monitor the most relevant, disease-driving immunological processes in different phases of (pre-)RA. This approach should be informative of the underlying biology and identify a targetable part of the pathogenic (auto-)immune response. Autoreactive B cells have the potential to meet these requirements and to serve as biomarkers of ‘immunological imminence’ (disease onset/flares), ‘immunological disease activity’ and ‘immunological remission’ in individual patients. Therefore, we developed a stringent, multimeric, antigen-specific multicolor flow cytometry and cell sorting technique that allows us to identify and isolate autoreactive B cells as single cells from patients. We combine this approach with state-of-the art single cell technology (such as spectral flow cytometry and single cell mRNA sequencing) and a tool-box of methods to sequence B cell receptor repertoires, express monoclonal antibodies, study their (cross-)reactivities and molecular characteristics, and generate immortalized cell lines that express specific B cell receptors.
Using these technologies in the context of the above, we run several projects that focus on:
- Factors initiating and maintaining chronicity of autoreactive B cell activation;
- Autoreactive B cells and their characteristics as biomarkers of imminent disease and/or immunological remission;
- Modulation of autoreactive B cells with existing and novel therapeutic approaches.
Together, we work on our ultimate aim, the conversion of RA from a chronic, life-long debilitating disease to a manageable, single acute event in the life of patients.
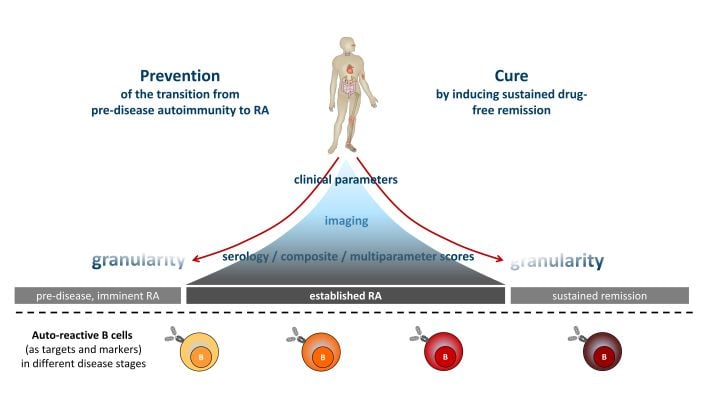
Conceptual embedding. Established RA (middle) can be controlled by treatment. Predicting disease onset (left) and inducing sustained remission (right), however, remain major hurdles in clinical care, despite increasing granularity from clinical parameters, imaging and serology. Attempts to prevent disease or to induce sustained remission are still largely empirical and rarely successful. We believe that autoreactive B cells can serve as functional biomarkers and targets that carry the biological information necessary to overcome these hurdles.
Projects
Themes for innovation / Societal Outreach
Key publications
Our team
Senior Scientist
- Hans Ulrich (Uli) Scherer, MD PhD
Scientific Team
- Prof. René EM Toes, PhD (Head of Experimental Rheumatology)
- Prof. Dr Tom W. J. Huizinga (Department Head)
- Linda Slot, MSc (Post-doc)
- Dr. Sanne Kampstra (Post-doc)
- Nienke Blomberg (PhD student)
- Sam Neppelenbroek (PhD student)
- Sophie-Anne Smith (PhD student)
- Sanne Kroos (PhD student)
- Sanne Reijm (PhD student)
- Aleida Bakker (Technician)
- Joanneke Kwekkeboom (Technician)
Senior Scientist
- Hans Ulrich (Uli) Scherer, MD PhD
Scientific Team
- Prof. René EM Toes, PhD (Head of Experimental Rheumatology)
- Prof. Dr Tom W. J. Huizinga (Department Head)
- Linda Slot, MSc (Post-doc)
- Dr. Sanne Kampstra (Post-doc)
- Nienke Blomberg (PhD student)
- Sam Neppelenbroek (PhD student)
- Sophie-Anne Smith (PhD student)
- Sanne Kroos (PhD student)
- Sanne Reijm (PhD student)
- Aleida Bakker (Technician)
- Joanneke Kwekkeboom (Technician)
Extended Scientific Team
- Dr. Diane van der Woude, MD PhD
- Dr. Karin van Schie, PhD
- Dr. Jolien Suurmond, PhD
- Dr. Cynthia Fehres, PhD
- Abdullelah Alzahrani (PhD student)
- Alice Bacon (PhD student)
- Roxane Biersteker (PhD student)
- Hugo van Dooren (PhD student)
- Miles Holborough-Kerkvliet (PhD student)
- Theresa Kissel (PhD student)
- Anouk van Mourik (PhD student)
- Wieke van Oostveen (PhD student)
- Sophie-Anne Smith (PhD student)
- Eva Maria Stork (PhD student)
- Esther Vletter (PhD student)
- Lars van Vliet (PhD student)
- Tineke van Wesemael (PhD student)
- Renee van de Wetering (PhD student)
Technical staff
- Annemarie Dorjee
- Nivine Levarth
- Gerrie Stoeken-Rijsbergen
- Ellen van der Voort