Klinische Chemie en Laboratoriumgeneeskunde
Dienstverlening
Alle praktische informatie over bepalingen die de afdeling Klinisch Chemie en Laboratoriumgeneeskunde verricht (o.a. referentiewaarden, de wijze van aanvragen, inzenden en doorlooptijden) is beschikbaar in de
Het document Historische referentiewaarden bevat een overzicht van huidige en historische referentiewaarden. Indien de kolom ‘geslacht’ is ingevuld, betreffen het geslachtsafhankelijke referentiewaarden. Indien de
Aanvullende informatie is te vinden in de ‘Wie doet wat database’ van de NVKC. De voorwaarden m.b.t. het insturen van materialen vindt u in de Dienstverleningsovereenkomst Afdeling KCL.
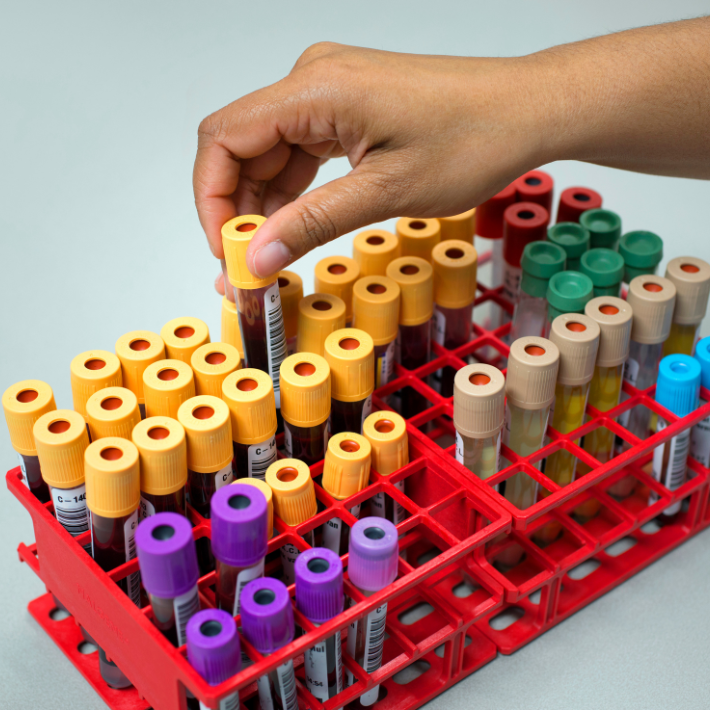
Afdeling KCL, van afname tot afvalbak
Video
In een animatie leggen we meer uit over het diagnostische testproces afdeling KCL.
&width=826&height=464)
Aanvragen en rapportage
Aanvragen
Externe ziekenhuizen kunnen gebruik maken van de extramurale aanvraagformulieren voor bloed, urine of overige materialen. Op deze aanvraagformulieren zijn de meest frequent aangevraagde bepalingen door externe instellingen opgenomen. De verzendcondities voor materialen van buiten het LUMC staan op de extramurale aanvraagformulieren vermeld.
Het LUMC werkt voor het aanvragen van laboratoriumdiagnostiek niet met ZorgDomein en heeft geen aanvraagformulieren voor eerstelijns aanvragers, met uitzondering van het aanvraagformulier voor neonatale bilirubine door verloskundigen.
De eerstvolgende Hypocretine analyse in liquor staat gepland voor de week van 23 februari 2026, waarna rapportage van de uitslagen binnen 14 dagen zal volgen. De volgende Hypocretine analyse staat gepland voor mei 2026.
Rapportage
Binnen het LUMC worden resultaten gerapporteerd in het Ziekenhuis Informatie Systeem HiX. Resultaten voor instellingen buiten het LUMC worden, zodra alle uitslagen binnen een aanvraag bekend zijn, d.m.v. een papieren rapportage verzonden naar het adres waarop de instelling staat ingeschreven in Vektis. Mocht u de uitslagen liever per (Secure) mail ontvangen i.p.v. de regulieren papieren rapportages, dan kunt u contact opnemen met laboratorium.kcl@lumc.nl.
De afdeling KCL hanteerd een lijst met sterk afwijkende waarden die direct worden doorgebeld aan de aanvrager, zie overzicht Ultrapathologische uitslagen. Voor aanvragers van buiten het LUMC hanteren wij dezelfde doorbellijst. Zorg daarom altijd dat een actueel telefoonnummer vermeld staat op uw aanvraag voor laboratoriumdiagnostiek.
Consultatie
Als aanvrager kunt u contact opnemen met de laboratoriumstaf over aanvragen en interpretatie van de uitslagen in relatie tot de klinische vraagstelling. Daarbij adviseert zij u als aanvrager over onderzoeksaanvragen, de relevantie ervan, de correlatie met andere aanvragen en de kosten ervan (preanalytisch consult). U krijgt ondersteuning bij de interpretatie van uitslagen in het licht van de afnameomstandigheden, biologische en analytische variatie en klinisch beeld van de patiënt (post-analytisch consult). De dienstdoende laboratoriumspecialist is 24/7 bereikbaar (zie contactgegevens). De laboratoriumstaf heeft de bevoegdheid aanvragen voor laboratoriumonderzoek al dan niet te honoreren.
Kwaliteitsborging
De afdeling Klinische Chemie en Laboratoriumgeneeskunde is door de Raad voor Accreditatie (RvA) geaccrediteerd volgens norm: NEN-EN-ISO-15189:2022 “Medische laboratoria – Bijzondere eisen voor kwaliteit en competentie”. Zie het accreditatiecertificaat Nederlands en Engels.
De scope (lijst met verrichtingen) waarvoor de accreditatie geldt, treft aan u op de website van de Raad voor Accreditatie.
De afdeling heeft een competentieverklaring betreffende haar competentie voor de uitvoering, analyse, interpretatie en rapportage van resultaten van diagnostisch onderzoek opgesteld. Voor vragen m.b.t. het kwaliteitssysteem kunt u terecht bij de kwaliteitsadviseur (zie contactgegevens).
Polikliniek
De afdeling Klinische Chemie en Laboratoriumgeneeskunde (KCL) heeft integrale opleidingsbevoegdheid voor het opleiden van laboratoriumspecialisten klinische chemie. De afdeling faciliteert stages voor HLO- en MLO-analisten i.o. en participeert in onderwijs voor verschillende groepen beroepsbeoefenaren zoals:
- Artsen in opleiding;
- Coassistenten;
- Verpleegkundigen;
- Analisten;
- Doktersassistenten;
- Klinisch perfusionisten in opleiding;
- Anesthesiemedewerkers in opleiding.
Opleidingen
Research groups (EN)
Laboratory Research Support
De unit LRS (Laboratory Research Support) binnen de afdeling Klinische chemie & Laboratoriumgeneeskunde (KCL) is uw aanspreekpunt voor vriesprotocollen, klinische trials en (biobank)studies. Wij kunnen het gehele proces (afname, analyse en opslag van lichaamsmaterialen) faciliteren. Bij iedere aanvraag hanteren wij een persoonlijke benadering waarbij u inhoudelijk advies krijgt van een van de laboratoriumspecialisten, om te verzekeren dat uw trial of studie altijd op correcte wijze wordt afgehandeld. Geen studie is hetzelfde, vanuit die gedachte profileert onze dienstverlening zich.
Contactgegevens
Bezoekadres
Vriendelijk verzoek u hier te melden wanneer u een afspraak heeft bij de afdeling KCL:
Locatie L-2-25
Route: 855
Medische post
Voor het toesturen van medische post kunt u gebruikmaken van ons antwoordnummer:
Antwoordnummer 10392
2300 WB Leiden
Postadres
Postzone E-2-P
Postbus 9600
2300 RC Leiden
Bezoekadres
Vriendelijk verzoek u hier te melden wanneer u een afspraak heeft bij de afdeling KCL:
Locatie L-2-25
Route: 855
Medische post
Voor het toesturen van medische post kunt u gebruikmaken van ons antwoordnummer:
Antwoordnummer 10392
2300 WB Leiden
Postadres
Postzone E-2-P
Postbus 9600
2300 RC Leiden
Dienstdoende laboratoriumspecialist
06 25 28 36 44
Uitslagen opvragen
071 526 41 37 (tijdens kantoortijden tussen 8.30 uur tot 17.00 uur)
Laboratorium
E-mail: Laboratorium.kcl@lumc.nl
071 526 22 51
Bloedafname
E-mail: Bloedafname.kcl@lumc.nl
071 526 57 55
Bloedtransfusie laboratorium
E-mail: Bloedtransfusie.kcl@lumc.nl
071 526 38 11
Coördinator Researchondersteuning (LRS)
E-mail: Trials.kcl@lumc.nl
071 529 82 99
Kwaliteit
E-mail: kwaliteit.kcl@lumc.nl
071 526 52 46
Secretariaat
E-mail: SecretariaatKCL@lumc.nl
071 526 22 78
Locatie E-2-21
Patiëntinformatie
Inzicht in uw medische gegevens
Het patiëntenportaal MijnLUMC helpt u met een duidelijk overzicht van uw behandeling en inzicht in uw medische gegevens.
Heeft u een klacht?
Uiteraard doen wij onze uiterste best om u zo goed mogelijk van dienst te zijn. Heeft u toch een suggestie of klacht, dan horen wij dit graag.
U kunt uw klacht indienen via het digitale klachten formulier.
Nieuwsbrieven
POCT en zelfmanagement
Enkele initiatieven voor Point of care testing en Zelfmanagement van Patiënten worden ondersteund. Meer informatie kunt u vinden onder Point of Care testing.
&width=710&height=710)
&width=180&height=180)