Ziekte Huidmodellen
In vitro huidziektemodellen
Weefselengineering betreft zowel het genereren van gezonde weefsels als zieke weefsels om verschillende pathologische aandoeningen beter te begrijpen. De oprichting van op menselijke cellen gebaseerde in vitro gemanipuleerde ziektemodelsystemen zou een paradigmaverschuiving kunnen vertegenwoordigen van inadequate conventionele monolaagcelculturen, of redelijk succesvolle diermodellen, naar meer fysiologisch weefselrelevante, patiëntspecifieke benaderingen.
…In vitro huidziektemodellen
Weefselengineering betreft zowel het genereren van gezonde weefsels als zieke weefsels om verschillende pathologische aandoeningen beter te begrijpen. De oprichting van op menselijke cellen gebaseerde in vitro gemanipuleerde ziektemodelsystemen zou een paradigmaverschuiving kunnen vertegenwoordigen van inadequate conventionele monolaagcelculturen, of redelijk succesvolle diermodellen, naar meer fysiologisch weefselrelevante, patiëntspecifieke benaderingen.
Dit concept van in vitro zieke huidmodellen wordt geïllustreerd door de modellen voor recessieve epidermolysis bullosa simplex (REBS) en cutaan plaveiselcelcarcinoom (SCC) die in ons laboratorium zijn ontwikkeld. REBS wordt gekenmerkt door ernstige intra-epidermale blaarvorming, als gevolg van de kwetsbaarheid van de basale keratinocyten die keratinetonofilamenten missen als gevolg van een homozygote nulmutatie in het keratine 14-gen. In vivo EBS-modellen, inclusief muizen- en hondenmodellen, zijn beperkt en vertegenwoordigen niet de menselijke micro-omgeving. Eerdere in vitro onderzoeken hebben een essentiële rol voor fibroblasten in het REBS-fenotype aangetoond; De beperkte beschikbaarheid van menselijke REBS-huidmonsters belemmerde echter verder in vitro onderzoek naar dermale-epidermale interacties in REBS. Om dit te ondervangen werd een explantatiebenadering gebruikt waarbij REBS-huidbiopten op een huidequivalent werden geplaatst waarin REBS-geassocieerde fibroblasten werden gezaaid.
Een vergelijkbare aanpak werd gebruikt om een in vitro model te construeren voor menselijke SCC, een kwaadaardige tumor van epidermale keratinocyten die wordt gekenmerkt door invasieve groei in de dermis. Na basaalcelcarcinoom is SCC de meest voorkomende maligniteit in blanke populaties met epidemische incidentiecijfers. Traditionele in vivo SCC-modellen zijn gebaseerd op het gebruik van chemische, genetische of mechanische inductie of voortplanting van carcinogenese bij muizen. De huidige in vitro benaderingen zijn beperkt tot het gebruik van cellijnen, die vaak geen waarheidsgetrouwe weergave van primaire huidkanker geven. De ontwikkeling van een representatief in vitro huidcarcinoommodel op basis van primaire SCC-biopten zorgt voor een beter begrip van fundamentele carcinogenesemechanismen en kan dienen als een gevalideerd pre-screeningplatform voor kandidaat-medicijnen, waardoor de behoefte aan dieren voor deze doeleinden wordt uitgeroeid.
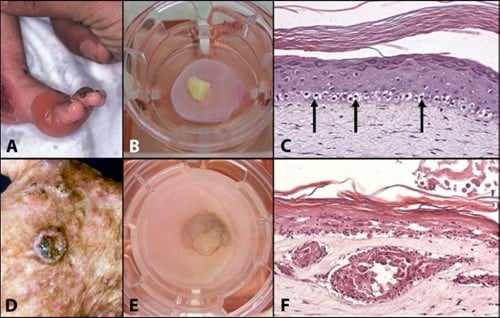
Figuur 1: Mimicking skin diseases in-vitro: (A) Clinical manifestation of Recessive Epidermolysis Bullosa Simplex (Blister disease); (B) REBS biopsy cultured onto the skin model; (C) Cross section of a skin model in which REBS has been mimicked; (D) Clinical manifestation of a squamous cell carcinoma; (E) SCC cultured onto the skin model; Cross section of a skin model mimicking SCC.
Projecten
- ZONMW: “Ontwikkeling van gevalideerde organotypische in vitro modellen die leiden tot verbeterde therapie van huidkanker”. Projectleiders, Frank de Gruijl, Kees Tensen en A El Ghalbzouri.
- STW: "Generatie van een gereconstrueerd huidmodel voor atopische dermatitis als hulpmiddel bij het screenen op therapieën”. In samenwerking met Prof.dr.J.Bouwstra, Faculteit der Natuurwetenschappen, Wiskunde en Informatica, Leiden/Amsterdam Centre for Drug Research, Drug Delivery Technology.
Huidmodellen als alternatief voor dierproeven
De algemene missie van de EU is ervoor te zorgen dat de gezondheid en veiligheid van mensen op het werk naar behoren worden gewaarborgd. De informatie waarop regelgevingsbeslissingen met betrekking tot de menselijke gezondheid zijn gebaseerd, is echter gedeeltelijk afkomstig van onderzoeken die bij proefdieren zijn uitgevoerd. Het zevende amendement van de Cosmeticarichtlijn is in september 2004 in nationaal recht omgezet. Het is een nieuwe richtlijn die betrekking heeft op het testen van cosmetische eindproducten en ingrediënten. De belangrijkste regels voor de nabije toekomst zijn:
- Met ingang van 11 maart 2009; een verbod op dierproeven met afgewerkte cosmetische ingrediënten binnen de EU.
- Vanaf maart 2009; een verbod op alle gevolgen voor de menselijke gezondheid, met uitzondering van toxiciteit bij herhaalde dosering, reproductietoxiciteit en toxicokinetiek. Voor deze specifieke gezondheidseffecten zal het verbod op het in de handel brengen stapsgewijs van toepassing zijn zodra alternatieve methoden zijn gevalideerd en in de EU-wetgeving zijn opgenomen, met inachtneming van het OESO-validatieproces, maar met een maximale uiterste datum van 10 jaar na de inwerkingtreding van het OESO-verdrag. de richtlijn, d.w.z. 11 maart 2013, ongeacht de beschikbaarheid van alternatieve dierproeven.
Alles bij elkaar laat dit duidelijk zien dat er behoefte is aan gevalideerde in vitro methoden voor het testen van de toxiciteit van chemicaliën, b.v. huidsensibilisatoren. Vele jaren geleden zijn onderzoekers begonnen met het zoeken naar alternatieve methoden. De belangrijkste stappen in het huidsensibilisatieproces (detectie, opname en verwerking van allergeen, cytokinesignalering, migratie/rijping van antigeenpresenterende cellen (DC's), activering en proliferatie van T-cellen) zijn gebruikt als uitleesparameters in deze testen. Hoewel sommige tests er veelbelovend uit zien, zijn ze nog lang niet perfect.
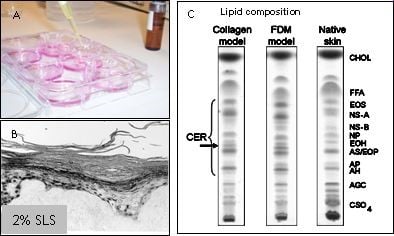
Figuur 2: HSE's worden gebruikt om verbindingen te testen op huidirritatie, huidcorrosie of huidsensibilisatie (A). De epidermis komt los van het dermale gedeelte wanneer 2% SLS plaatselijk op de HSE wordt aangebracht (B). De HSE bevat een competente barrière, weergegeven in een dunnelaagchromatografieprofiel van de lipidensamenstelling van HSE's en de natuurlijke huid (C).
Hoewel de huidmodellen voor een groot deel de barrière-eigenschappen van de normale menselijke huid reproduceren, zijn er toch enkele kleine verschillen aanwezig. Deze verschillen kunnen de voorspelbaarheid van een geteste verbinding beïnvloeden. In een samenwerkingsproject uitgevoerd bij het LACDR willen we de barrière-eigenschappen van de huidmodellen verder verbeteren door de lipidensamenstelling in het stratum corneum te wijzigen. In eerdere onderzoeken hebben we aangetoond dat LEM's betrouwbare en produceerbare epidermale modellen zijn die geschikt zijn voor de screening van potentiële huidirriterende stoffen. De teststoffen kunnen topisch worden toegepast en hun irriterend potentieel kan worden geëvalueerd aan de hand van verschillende eindpunten, zoals de inductie van weefselbeschadiging of de afgifte van verschillende pro-inflammatoire mediatoren, veranderingen in eiwit- en mRNA-expressieprofielen. Een soortgelijke aanpak kan worden gevolgd voor het testen van andere verbindingen, zoals corrosieve verbindingen. Studies met HSE's kunnen daarom bijdragen aan onze kennis over de fundamentele biochemische mechanismen die ten grondslag liggen aan irriterende reacties, en kunnen worden gebruikt om de structurele kenmerken van moleculen te begrijpen, die mogelijk verantwoordelijk zijn voor het uitlokken van een irriterende reactie. Bovendien maakt het genereren van epidermale equivalenten, bevolkt met zowel keratinocyten als melanocyten, het mogelijk om de regulatie van melanogenese, melanocyt-keratinocyt-interacties en hoe deze processen worden beïnvloed door UV-straling te bestuderen. Een dergelijk model kan ook worden gebruikt voor het testen van de fototoxische of fotobeschermende eigenschappen van verschillende verbindingen en zonnebrandmiddelen. In een ander samenwerkingsproject met het RIVM verwachten we biomarkers te identificeren die kunnen worden gebruikt om huidsensibilisatoren te onderscheiden van huidirriterende stoffen om zo eenvoudige en betrouwbare testsystemen te ontwikkelen. Hiervoor zullen we verschillende celtypen gebruiken, waaronder cellijnen, primaire keratinocyten, dendritische cellen en/of epidermale huidmodellen. Daarnaast zullen signaaltransductieroutes waarvan bekend is dat ze een belangrijke rol spelen bij huidsensibilisatie, zoals de KEAP1/Nrf2-route, diepgaand bestudeerd worden om het huidsensibilisatieproces beter te begrijpen.
Projecten
- LACDR: "Verbetering van de barrière-eigenschappen in gereconstrueerde menselijke huidmodellen”. In samenwerking met Prof.dr.J.Bouwstra.
- Nederlands Toxicologisch Centrum (NTC): “Validatie van moleculaire markers van huidsensibilisatie door gene silencing in menselijke keratinocyten en 3D gereconstrueerde huidmodellen”. In samenwerking met prof.dr. H van Loveren (RIVM).
In vitro nabootsen van huidaandoeningen (bijvoorbeeld verwondingen, veroudering)
Omdat menselijke huidmodellen eenvoudig te moduleren zijn, kunnen verschillende huidaandoeningen zoals littekenvorming, huidveroudering en wondgenezing worden nagebootst en bestudeerd. Voor dit doel kunnen cellen afkomstig van huidweefsels die een bepaalde fysiologische aandoening of ziekte vertegenwoordigen, worden geïsoleerd, gekweekt en in het menselijke huidmodel worden opgenomen. Door cellen te isoleren uit huidweefsel verkregen van jonge en oudere individuen kunnen we bijvoorbeeld verschillende processen nabootsen die optreden tijdens huidveroudering (bijvoorbeeld dunner worden van de opperhuid, afname van de collageenafzetting). De meeste van deze processen worden uitgebreid bestudeerd in muismodellen of in conventionele monocellagen.
Door gebruik te maken van menselijke huidmodellen kunnen we een micro-omgeving nabootsen die sterk lijkt op die van het in vivo menselijke weefsel, in tegenstelling tot conventionele monolaagculturen en muismodellen. Cellen van oudere individuen worden vaak gekenmerkt door onjuiste eiwitexpressie, zoals opregulatie van collagenase en neerwaartse regulatie van de collageenproductie. Cellen die in vitro "verouderd" zijn, vertonen vergelijkbare kenmerken (bijvoorbeeld lage afzetting van collageen I, celveroudering, apoptose, vertraagde wondgenezing). Door de huidmodellen bloot te stellen aan UV kunnen we bovendien processen bestuderen die betrokken zijn bij fotoveroudering, door bijvoorbeeld te kijken naar DNA-schade. Omdat huidmodellen gemakkelijk te hanteren zijn, kunnen verschillende wonden worden geïntroduceerd. Door een klein fragment van de epidermis te verwijderen, ontstaat er een oppervlakkige wond. Het is ook mogelijk om wonden van volledige dikte in te brengen om brandwonden na te bootsen. Met deze aanpak kunnen we processen onderzoeken die plaatsvinden bij bijvoorbeeld brandwondenpatiënten. Brandwondenpatiënten zijn een belangrijk doelwit voor infecties met hun open wonden en lange ziekenhuisverblijven. Onlangs is gemeld dat orgaanfalen in meerdere systemen (waarvoor in 45,9% van de gevallen een infectie verantwoordelijk was) de meest voorkomende doodsoorzaak is bij patiënten met brandwonden.
Bovendien is een chirurgische huidtransplantatie de gebruikelijke manier om de verbrande huid te herstellen, maar bij bacteriële kolonisatie/infecties wordt een dergelijke procedure vaak uitgesteld. Bovendien reageren de infectieuze agentia mogelijk niet optimaal op de behandeling met antibiotica, omdat er vaak antibioticaresistente stammen worden aangetroffen en/of bacteriën biofilms kunnen vormen. In een samenwerkingsproject met de afdeling infectieziekten zullen we de moleculaire basis onderzoeken van de interacties tussen bacteriën (biofilms) en menselijke cellen in (thermisch beschadigde) menselijke huidmodellen die samen met bacteriën worden gekweekt. Dit geeft ons de mogelijkheid om een in vitro model te ontwikkelen waarmee de effecten van experimentele medicatie, antibiotica en/of antimicrobiële peptiden kunnen worden bepaald.
Projecten
- Cosmetische en farmaceutische industrie: “Identificatie van biomarkers voor huidveroudering met behulp van gereconstrueerde menselijke huidmodellen”.
- Nederlandse Brandwonden Stichting (NBS): "Nieuwe strategieën voor de preventie en behandeling van brandwondinfecties in huidmodellen". In samenwerking met Dr. P.Nibbering, Infectieziekte, LUMC.

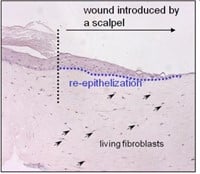
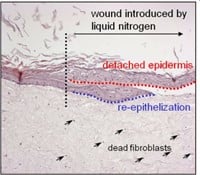
Figuur 3: HSE is gewond geraakt met een diepe wond (vloeibare stikstof) of oppervlakkige wond (scalpel). Er wordt een nieuwe epidermis gevormd die actief is bij migratie en proliferatie, zoals aangetoond met een hyperprolifertaion-geassocieerde marker Keratin 17 in (bovenste afbeelding).
Verschillende soorten menselijke huidmodellen
Binnen onze afdeling lopen diverse projecten waarbij gebruik wordt gemaakt van verschillende soorten menselijke huidequivalenten. Afhankelijk van de onderzoeksvragen kunnen de volgende huidmodellen worden ontwikkeld: Leiden Epidermal model (LEM), het Full-Thickness model (FTM) en het Fibroblasts-Derived Matrix model FDM). 1) LEM bestaat uit keratinocyten die zijn gezaaid op een niet-cellulaire matrix (bijvoorbeeld een inert filtermembraan of gede-epidermische dermis. Deze epidermale modellen zijn geschikt voor bijvoorbeeld corrosiviteits-, irritatie- of penetratietests. 2) FTM bestaat uit keratinocyten, melanocyten en een fibroblast -bevolkte driedimensionale collageenmatrix. Dit model lijkt sterk op de inheemse menselijke huid. Dit huidmodel met volledige dikte kan worden gebruikt voor tests, voorspellende screening en onderzoek naar bijvoorbeeld wondgenezing waarvoor de complexiteit van de menselijke huid vereist is, d.w.z. waarbij de interactie tussen epidermale en dermale cellen cruciaal is. 3) FDM is vergelijkbaar met het FTM-model, maar het dermale compartiment bestaat uit van menselijke fibroblasten afgeleide extracellulaire matrix. Dit model kan worden gebruikt als hulpmiddel om het effect van b.v. ingrediënten op dermale processen. Alle drie de typen kunnen worden gebruikt voor voorspellende screening of contractonderzoek waarvoor de complexiteit van de menselijke huid vereist is. Variaties op deze modellen kunnen worden gegenereerd door de integratie van andere celtypen (bijvoorbeeld endotheelcellen). Modellen kunnen ook worden gekweekt bij verschillende vochtigheids- en zuurstofconcentraties.
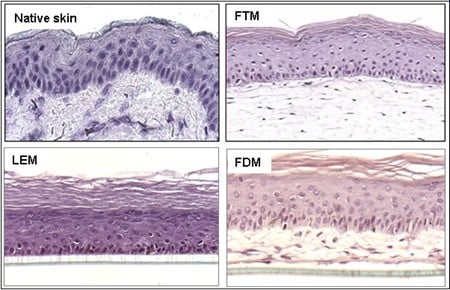
Figuur 4: Verschillende soorten huidmodellen.
Hoofdonderzoeker
Dr. A. El Ghalbzouri
Menselijke huidequivalenten (HSE) zijn driedimensionale systemen die worden ontwikkeld door fibroblasten in een driedimensionale huidmatrix te plaatsen. Na specifieke kweekomstandigheden wordt een HSE gevormd die de meeste in vivo kenmerken recapituleert en waarin cellulaire processen kunnen worden genormaliseerd in vergelijking met de conventionele monolaagculturen. HSE is daarom een aantrekkelijk hulpmiddel voor het bestuderen van cel-cel-, cel-matrix-, dermale-epidermale interacties en andere processen die betrokken zijn bij epidermale morfogenese. Daarnaast zijn de HSE ook een uitstekend hulpmiddel om zieke huidaandoeningen in vitro na te bootsen (bijvoorbeeld Epidermolysis Bullosa Simplex en Plaveiselcelcarcinoom) om therapieën te testen.


Figuur 5: Getoond wordt een (links) en een dwarsdoorsnede van de gekweekte huid (rechts).