Klinische Farmacie en Toxicologie
&width=710&height=710)
Op de afdeling Klinische Farmacie en Toxicologie van het LUMC werken ruim 200 betrokken professionals. Dagelijks zetten zij zich binnen de ziekenhuisapotheek in voor het maken en leveren van geneesmiddelen voor patiënten van het LUMC en daarbuiten. Daarnaast dragen zij zorg voor het veilig gebruik ervan.
De ziekenhuisapotheek van het LUMC is specialist in gepersonaliseerde geneesmiddelenzorg en zoekt met veel passie en kunde naar de best passende behandeling voor elke patiënt.
Farmaceutische patiëntenzorg
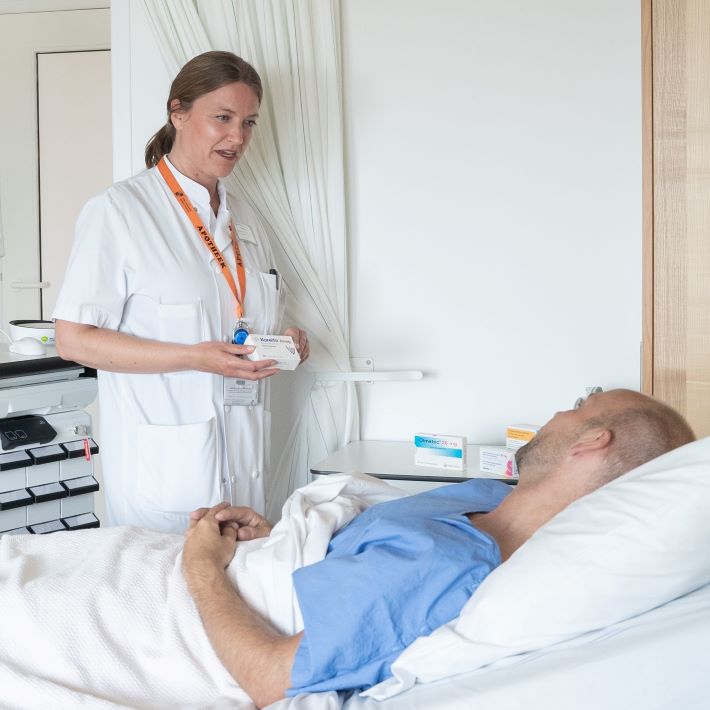
Binnen de Farmaceutische patiëntenzorg staat de patiënt centraal. Voor alle patiënten die in het LUMC zijn opgenomen, brengen onze apothekersassistenten de thuismedicatie in kaart. Vervolgens maken ze de medicatie klaar die u in het ziekenhuis toegediend krijgt.
Alle medicatie die in het ziekenhuis wordt voorgeschreven, wordt gecontroleerd op dosering en wisselwerkingen met andere medicatie. De ziekenhuisapothekers zijn betrokken bij het opstellen van deze medicatieprotocollen. Zij nemen ook deel aan de multidisciplinaire overleggen in het LUMC. Daarnaast voeren de ziekenhuisapothekers medicatiereviews uit, waarbij gekeken wordt of de juiste medicatie in de juiste dosering gebruikt wordt.
…Farmaceutische patiëntenzorg
Binnen de Farmaceutische patiëntenzorg staat de patiënt centraal. Voor alle patiënten die in het LUMC zijn opgenomen, brengen onze apothekersassistenten de thuismedicatie in kaart. Vervolgens maken ze de medicatie klaar die u in het ziekenhuis toegediend krijgt.
Alle medicatie die in het ziekenhuis wordt voorgeschreven, wordt gecontroleerd op dosering en wisselwerkingen met andere medicatie. De ziekenhuisapothekers zijn betrokken bij het opstellen van deze medicatieprotocollen. Zij nemen ook deel aan de multidisciplinaire overleggen in het LUMC. Daarnaast voeren de ziekenhuisapothekers medicatiereviews uit, waarbij gekeken wordt of de juiste medicatie in de juiste dosering gebruikt wordt.
De ziekenhuisapotheek is een belangrijke vraagbaak voor de artsen, verpleegkundigen en apothekers uit de regio. Maar ook patiënten zijn welkom om hun vragen direct te stellen aan de ziekenhuisapothekers van het LUMC. Dit kan via ‘vraag aan de apotheker’ in de medicatierubriek van MijnLUMC.
Farmaceutische bereidingen
De afdeling Klinische Farmacie en Toxicologie worden tal van geneesmiddelen op grote schaal en onder de hoogst geldende kwaliteitsnormen geproduceerd. De farmaceutische bereidingen zijn bestemd voor patiënten binnen het LUMC, maar ook voor daarbuiten.
Voor individuele patiënten maakt de afdeling geneesmiddelen op maat bereid, zoals cytostatica, radiofarmaca, parenterale voedingen. Ook kan de ziekenhuisapotheek helpen om dosisvormen aan te passen, om zo een behandeling waarvoor geen bestaand geneesmiddel beschikbaar is, toch mogelijk te maken. Dit geldt onder andere voor specifieke patiëntengroepen, zoals kinderen, waarvoor veelal geen geschikte sterkte van het geneesmiddel beschikbaar is.
De ziekenhuisapotheek van het LUMC maakt daarnaast ook nog experimentele geneesmiddelen in het kader van onderzoek.
Klinisch Farmaceutisch Laboratorium
In het laboratorium binnen de ziekenhuisapotheek in het LUMC controleren we de kwaliteit en veiligheid van geneesmiddelen. Op basis van DNA-onderzoek en het meten van geneesmiddelconcentraties in het bloed geven we advies over de juiste keuze en dosering van geneesmiddelen. Hierdoor helpt de ziekenhuisapotheek de behandeling van de patiënt op maat te maken. Er vindt hoogwaardig wetenschappelijk klinisch farmaceutisch laboratoriumonderzoek plaats. En het laboratorium is continu op zoek naar nieuwe methoden om de behandeling met geneesmiddelen te verbeteren.
Het Klinisch Farmaceutisch Laboratorium van het LUMC is GMP- en ISO15189-gecertificeerd.
Producten en diensten aan externen
Zorginstellingen en apotheken kunnen bij ons geneesmiddelen bestellen die door de ziekenhuisapotheek van het LUMC op voorraad worden bereid.
Tevens kunnen externen laboratoriumdiagnostiek op het gebied van TDM/Toxicologie en Farmacogenetica aanvragen bij ons Klinisch Farmaceutisch Laboratorium.
Producten en diensten aan externen
Lees meer over bestellen van geneesmiddelen en het aanvragen van labdiagnostiek voor externen.
Polikliniek Apotheek
De poliklinische apotheek van het LUMC heeft een ruim assortiment geneesmiddelen, afgestemd op de specialistische behandelingen van het LUMC. Na opname, dagbehandeling of bezoek aan de polikliniek, haal je hier als patiënt je medicijnen op. Ook verbandproducten, hulpmiddelen en zelfzorgmiddelen zijn verkrijgbaar.
Polikliniek Klinische Farmacologie
Deze polikliniek wordt gecoördineerd door de afdeling Interne Geneeskunde en de ziekenhuisapotheek van het LUMC. De polikliniek is er voor patiënten met een klinisch farmacologisch probleem. Gedacht kan worden aan patiënten met ernstige of bijzondere bijwerkingen, aan patiënten bij wie een farmacogenetische test moet worden verricht of aan patiënten met polyfarmacie.
De afdeling Klinische Farmacie en Toxicologie leiden de (ziekenhuis)apothekers, behandelaars en voorschrijvers van de toekomst op, die in de praktijk staan. Immers, de farmaceutische zorg wordt complexer en de patiënt meer veeleisend. Wij coördineren en verzorgen onderwijs, bij- en nascholing op het gebied van geneesmiddelen, klinische farmacologie, farmacotherapie en geneesmiddeltoxicologie.
Opleidingen
Drug treatment is the cornerstone of today’s medicine. In this respect, pharmacology is a key driver for the advancement of health care and thus essential for patient’s well-being. However, the outcome of drug treatment is highly variable. Indeed, everyday large numbers of patients receive medications that are, at an individual level, either ineffective or cause side effects. Therefore, to successfully treat individual patients a personalised therapeutics approach is needed.
…Drug treatment is the cornerstone of today’s medicine. In this respect, pharmacology is a key driver for the advancement of health care and thus essential for patient’s well-being. However, the outcome of drug treatment is highly variable. Indeed, everyday large numbers of patients receive medications that are, at an individual level, either ineffective or cause side effects. Therefore, to successfully treat individual patients a personalised therapeutics approach is needed.
Personalised Therapeutics
Our Personalised Therapeutics research program is centered on three main themes:
- Discovery and implementation of pharmacogenomic biomarkers in clinical practice
- Discovery and development of personalised drug treatments
- Optimize drug use in individual patients using Real World Data
Contactgegevens
Postadres
Postbus 9600
2300 RC Leiden
Gebouw 1, route 833
Postzone L0-P
Secretariaat
kamer L0-215
e-mail: KFTsecretariaat@lumc.nl
tel.: +31 (0)71 52 62790
Dienstdoende ziekenhuisapotheker
tel. +31 (0)71 52 99758 (tijdens kantooruren)
tel. +31 (0)71 52 69111 (buiten kantooruren)
Postadres
Postbus 9600
2300 RC Leiden
Gebouw 1, route 833
Postzone L0-P
Secretariaat
kamer L0-215
e-mail: KFTsecretariaat@lumc.nl
tel.: +31 (0)71 52 62790
Dienstdoende ziekenhuisapotheker
tel. +31 (0)71 52 99758 (tijdens kantooruren)
tel. +31 (0)71 52 69111 (buiten kantooruren)
Polikliniek Apotheek
e-mail: Poliapotheek@lumc.nl
tel. +31 (0)71 52 98531 (tijdens kantooruren)
Polikliniek Klinische Farmacologie
e-mail: internegeneeskunde@lumc.nl
tel. +31 (0)71 52 98150 (09.00 tot 12.00 uur)
Aanvragen contracten aan hoofd apotheek
Secretariaat (of email: K.J.M.Schimmel@lumc.nl)
Secretariaat Masteropleiding Farmacie
e-mail: secr.med.farm@lumc.nl
+31 (0)71 52 99894 (tijdens kantooruren)
Onze visie
De afdeling Klinische Farmacie en Toxicologie van het LUMC zoekt voor elke patiënt de beste medicamenteuze behandeling afgestemd op de persoonlijke behoeftes van de patiënt.
Wij zijn de specialist in gepersonaliseerde geneesmiddelenzorg. Door onderzoek leveren we een tastbare bijdrage aan betere farmaceutische patiëntenzorg, nu en in de toekomst.
Wij leiden, in een veilig opleidingsklimaat, de apothekers en artsen van de toekomst op, die in de praktijk staan. Immers, de zorg wordt complexer en de patiënt meer veeleisend.
…De afdeling Klinische Farmacie en Toxicologie van het LUMC zoekt voor elke patiënt de beste medicamenteuze behandeling afgestemd op de persoonlijke behoeftes van de patiënt.
Wij zijn de specialist in gepersonaliseerde geneesmiddelenzorg. Door onderzoek leveren we een tastbare bijdrage aan betere farmaceutische patiëntenzorg, nu en in de toekomst.
Wij leiden, in een veilig opleidingsklimaat, de apothekers en artsen van de toekomst op, die in de praktijk staan. Immers, de zorg wordt complexer en de patiënt meer veeleisend.
Gedreven, nieuwsgierig en vakmanschap zijn kenmerkende eigenschappen van mensen van onze afdeling. Samen werken we met liefde voor het vak aan de best passende behandeling voor elke patiënt. Wij zijn trots op ons werk, het innovatieve karakter van de afdeling en de beschikbare faciliteiten. Er wordt met veel plezier en passie gewerkt aan veilige en doelmatige patiëntenzorg, patiëntgericht en multidisciplinair onderwijs en grensverleggend onderzoek.
Kwaliteit van onze zorg
De afdeling Klinische Farmacie en Toxicologie is door IGJ GMP gecertificeerd en beschikt over een GPM-z erkenning. Ook het Klinisch Farmaceutisch Laboratorium voldoet aan de hoogste kwaliteitsstandaarden en is GMP- en ISO15189-gecertificeerd. De afdeling beschikt ook over een fabrikantenvergunning voor geneesmiddelen voor onderzoek. Deze vergunning staat toe dat klassieke geneesmiddelen, ATMPs, peptiden en radiofarmaca mogen worden gemaakt voor onderzoek. Het team kwaliteitsbeheer registreert voor de hele afdeling kwaliteitsindicatoren, die ingezet worden om de kwaliteit zichtbaar te maken. Wij streven naar de hoogst haalbare kwaliteit binnen alle facetten van de farmacie.
&width=180&height=180)
&width=180&height=180)