Interventional Nuclear Medicine
Practical interventional nuclear medicine implementations are exemplified by applications such as needle guidance (e.g., PET guided biopsy), focal therapy delivery (e.g., Radioembolization) and radioguided surgery (e.g., sentinel node biopsy).
Needle guidance
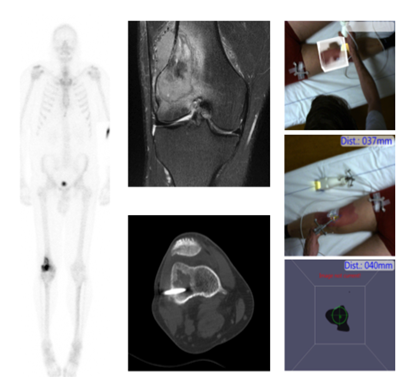
Identification of suspected disease via imaging is one thing, but confirming its pathology is another. In other words, it is important to obtain a representative biopsy to be able to demonstrate disease or the absence thereof. Acquiring a biopsy can be challenging in the absence of substrate on radiological examination or in case of (benign or malignant) inhomogeneous processes. Examples of such cases are, primary oncologic diseases (e.g., bone and soft tissue and breast cancer), small metastatic disease, and benign diseases like infection (e.g., spondylitis). To prevent a mismatch between imaging and pathology (in the form of false negative biopsies) lesions identified via for example PET/CT can be biopted under molecular-guidance. That way the need for repeated biopsies can be minimized.
…Needle guidance
Identification of suspected disease via imaging is one thing, but confirming its pathology is another. In other words, it is important to obtain a representative biopsy to be able to demonstrate disease or the absence thereof. Acquiring a biopsy can be challenging in the absence of substrate on radiological examination or in case of (benign or malignant) inhomogeneous processes. Examples of such cases are, primary oncologic diseases (e.g., bone and soft tissue and breast cancer), small metastatic disease, and benign diseases like infection (e.g., spondylitis). To prevent a mismatch between imaging and pathology (in the form of false negative biopsies) lesions identified via for example PET/CT can be biopted under molecular-guidance. That way the need for repeated biopsies can be minimized.
We are currently implementing molecular-guided biopsy in infectious diseases, bone lesions, and breast cancer. By increasing the imaging speed while lowering the radiation burden we work on facilitating on-scanner needle guidance. Next to that we are also studying the use of navigation and tracking systems to facilitate needle placement. Molecular Breast Imaging (MBI; also known as Breast-specific gamma imaging (BSGI)) guided are routinely implemented as problem solver in high-risk patients.
Focal therapy delivery
Imaging not only provides information with regard to target identification; it also provides a valuable means to simulate therapy delivery. A prime example of this is found in Selective Internal Radiation Therapy (SIRT) also known as Transarterial Radioembolization (TARE). In this procedure a diagnostic scout scan (99mTc-MAA) is used to identify shunting and perform dosimetry prior to the therapeutic delivery of e.g., 90Y- microspheres. A concept that currently sees application in HCC and mCRC.
…Focal therapy delivery
Imaging not only provides information with regard to target identification; it also provides a valuable means to simulate therapy delivery. A prime example of this is found in Selective Internal Radiation Therapy (SIRT) also known as Transarterial Radioembolization (TARE). In this procedure a diagnostic scout scan (99mTc-MAA) is used to identify shunting and perform dosimetry prior to the therapeutic delivery of e.g., 90Y- microspheres. A concept that currently sees application in HCC and mCRC.
In our efforts to advance treatment planning we reason that a better understanding of technical pitfalls and dosimetry will help improve patient outcomes. Hereby we strive to adequately treat the disease while sparing healthy tissue. Next to our efforts in SIRT, we are actively investigating possibilities to extend the paradigm to other local drug-delivery applications.
Radioguided surgery
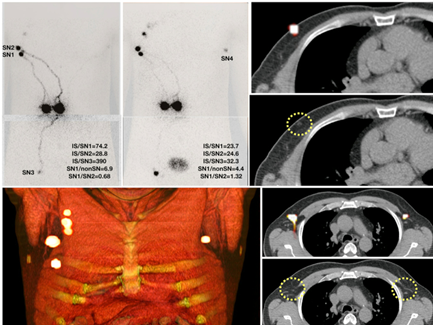
Surgery is increasingly relying on the use of imaging, a concept that is globally still dominated by radioguided surgery. Again, imaging roadmaps generated at the department of nuclear medicine help select patients for surgical treatment regimes. A timely example is the use of PSMA-PET scans to select patients that can benefit from PSMA-targeted radioguided surgery. But even with the roadmaps, it remains difficult to relate the preoperative findings to the actual surgery itself. A challenge that is especially prominent in anatomically difficult areas (around vessels and nerves) such as head-and-neck and pelvic area. The specificity and sensitivity of intraoperative nuclear tracing and imaging helps to overcome this challenge.
…Radioguided surgery
Surgery is increasingly relying on the use of imaging, a concept that is globally still dominated by radioguided surgery. Again, imaging roadmaps generated at the department of nuclear medicine help select patients for surgical treatment regimes. A timely example is the use of PSMA-PET scans to select patients that can benefit from PSMA-targeted radioguided surgery. But even with the roadmaps, it remains difficult to relate the preoperative findings to the actual surgery itself. A challenge that is especially prominent in anatomically difficult areas (around vessels and nerves) such as head-and-neck and pelvic area. The specificity and sensitivity of intraoperative nuclear tracing and imaging helps to overcome this challenge.
Sentinel Node is a text book example of a globally accepted radioguided surgery indication. Currently rapid adaptation of receptor specific radioguided surgery applications has emerged. In this field our group introduces new enabling technologies and tries to refine existing procedures. An example is the integration of fluorescence detection and navigation in radioguided surgery workflows.
Themes for innovation
Key Publications
Our Team
- Daphne D.D. Rietbergen (PI, MD, PhD researcher)
- Fijs W.B. van Leeuwen, (PI and Professor Molecular imaging and image guided therapy)
- Renato A. Valdes Olmos (MD)
- Tessa Buckle, (PI, Professor Translational image-guided surgery)
- Matthias N. van Oosterom, Engineer Medical Technologies (PI, postdoc)
- Frits Smit (MD)
- Lenka M. Pereira Arias-Bouda (PI, MD)
- Samaneh Azghargoshab (PhD researcher)
- Imke Boekenstein (PhD researcher)