Muscle and Synapse Disorders
The LUMC is national clinical referral centre for all of these conditions, and member of the European Reference Network EURO-NMD.
Focus and Aim
Our focus is on disorders in which the LUMC conducts translational research and provides dedicated multidisciplinary care for children and adults. The aim is to understand the pathophysiology and clinical variability in these rare conditions, to identify targets and strategies for treatment, and ultimately to provide innovative therapies for our patients.
LUMC Biobank for neuromuscular disorders
Understanding neuromuscular disorders (NMD) is crucial for tailored care and assessing new treatments. The LUMC NMD Biobank serves as a valuable resource for evaluating disease progression and symptoms, uncovering potential biomarkers, and aids in identifying genetic and epigenetic factors. Its main aim is to gain novel insights to improve care and treatment of individuals with NMD.
With patients consent, the LUMC NMD Biobank collects longitudinal biomaterials and clinical data from a wide range of patients with NDM spanning various age groups. Specifically for patients diagnosed with Duchenne or Becker muscular dystrophy, clinical data is collected at the source in a standardized manner, with data elements that are unambiguously defined with internationally adopted ontologies. To achieve this, FISMA (Framework for Information Specification, Modeling, and Architecture) is integrated into the electronic healthcare system (EHS). There are plans to broaden its reach to additional NMD within EHS. For more information on FISMA, visit the Dutch Dystrophinopathy Dataset webpage.
…Understanding neuromuscular disorders (NMD) is crucial for tailored care and assessing new treatments. The LUMC NMD Biobank serves as a valuable resource for evaluating disease progression and symptoms, uncovering potential biomarkers, and aids in identifying genetic and epigenetic factors. Its main aim is to gain novel insights to improve care and treatment of individuals with NMD.
With patients consent, the LUMC NMD Biobank collects longitudinal biomaterials and clinical data from a wide range of patients with NDM spanning various age groups. Specifically for patients diagnosed with Duchenne or Becker muscular dystrophy, clinical data is collected at the source in a standardized manner, with data elements that are unambiguously defined with internationally adopted ontologies. To achieve this, FISMA (Framework for Information Specification, Modeling, and Architecture) is integrated into the electronic healthcare system (EHS). There are plans to broaden its reach to additional NMD within EHS. For more information on FISMA, visit the Dutch Dystrophinopathy Dataset webpage.
Data and sample collection
At March 2024, the LUMC NMD Biobank contains de-identified data and biological samples from 846 participants with a NMD. Biomaterials, including DNA, serum, plasma, urine, and pax tubes for RNA, are available in a longitudinal manner, offering a wealth of information for research purposes. For more detailed information of the numbers, please refer to the graphs provided below.
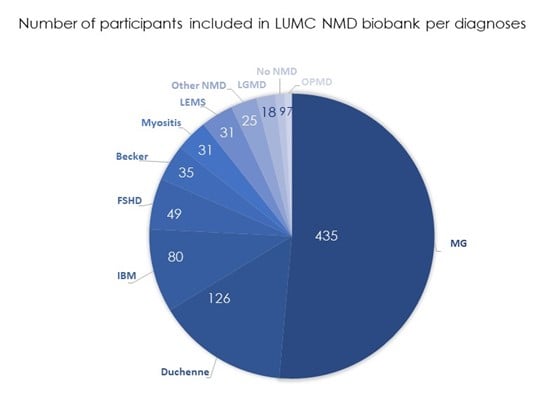
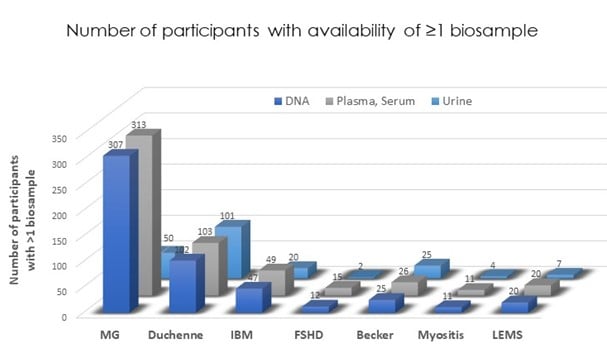
Inclusion in the NMD Biobank
Neuromuscular doctors at the LUMC will inform their patients about the NMD Biobank.
Data access for researchers
The NMD Biobank is open to approved researchers. By sharing biological samples, we aim to facilitate research toward effective treatments.
Are you a researcher outside the LUMC NMD team, please first contact the NMD team to collaborate via email: Neuromuscularcentre-Leiden@lumc.nl
Are you a researcher within the LUMC NMD team, follow the steps below to use biomaterials and/or associated clinical data:
- Reach out to the NMD Biobank team via Neuromuscularcentre-Leiden@lumc.nl to confirm the availability of your sample of interest
- After confirmation submit a written LUMC research biobank protocol, assess by the Science Committee of the relevant department
- After approval, undergo assessment by the LUMC review Committee on Biobanks and Biomaterials (BTC@LUMC.nl). Check TCBio for the required documents.
- When material stored at the Biobank must be issued, send the approved research protocol to Biobankorganisatie@lumc.nl and inform the NMD Biobank team via Neuromuscularcentre-Leiden@lumc.nl. The biobank organization will support the biomaterial release.
Check the LuVDS page if you’re interested in using material from healthy volunteers.
Join us in advancing research and finding effective treatments for neuromuscular disorders!
Contact at: Neuromuscularcentre-Leiden@lumc.nl
Duchenne & Becker Muscular Dystrophy (DMD/BMD)
LUMC is partner of the Duchenne Centre Netherlands (DCN), a collaboration between LUMC, Radboudumc, the Centre for neurological learning disorders Kempenhaeghe, and the Dutch patient representative organisations Duchenne Parent Project and Spierziekten Nederland. DCN governs the national patient registry, the Dutch Dystrophinopathy Database. Through the National biobank project DCN co-works with 5 other university medical centres and 4 centres for home respiratory ventilation to collect data from clinical care to study genetic and circulating biomarkers. Biobanking is completely integrated into our outpatient clinical care. Learning and behaviour of DMD/BMD patients is being studied as part of the EU Horizon 2020 program Brain Involvement iN Dystrophinopathies (BIND) and includes advanced MRI techniques.
…Duchenne & Becker Muscular Dystrophy (DMD/BMD)
LUMC is partner of the Duchenne Centre Netherlands (DCN), a collaboration between LUMC, Radboudumc, the Centre for neurological learning disorders Kempenhaeghe, and the Dutch patient representative organisations Duchenne Parent Project and Spierziekten Nederland. DCN governs the national patient registry, the Dutch Dystrophinopathy Database. Through the National biobank project DCN co-works with 5 other university medical centres and 4 centres for home respiratory ventilation to collect data from clinical care to study genetic and circulating biomarkers. Biobanking is completely integrated into our outpatient clinical care. Learning and behaviour of DMD/BMD patients is being studied as part of the EU Horizon 2020 program Brain Involvement iN Dystrophinopathies (BIND) and includes advanced MRI techniques.
To unravel the natural history and disease modifiers several national and international natural history studies in DMD and BMD are ongoing. Specific focus is on the long-term influence of corticosteroid treatment on muscle function and bone health.
LUMC develops muscle MRI as biomarker to quantify muscle changes over time and to relate these to clinically important differences in both DMD and BMD.
LUMC facilitates numerous international clinical trials focusing on muscle regeneration, alternative steroid treatment, and modification of RNA or DNA.
Limb Girdle Muscular Dystrophy
LUMC is the national referral center for LGMD in the Netherlands. A national patient registry has gone online in 2022 in collaboration with the Dutch patient organization Spierziekten Nederland. Aim is to study the epidemiology of this variety of genetic conditions, to collect natural history data, and to be able to facilitate recruitment for clinical studies. We are currently preparing the first clinical trials in LGMD.
Facioscapulohumeral Dystrophy (FSHD)
Studies in FSHD are done in close collaboration with the Department of Human Genetics, within the FSHD expert centre, a partnership between the LUMC and Radboud UMC. To test whether molecular diagnosis of FSHD could be simplified by testing of urine or skin a study on disease properties is being done in these. To enhance safety for upcoming DUX4 targeting drugs the function of DUX4, the genetic product underlying the disease, is being investigated in the thymus where DUX4 is expressed as well. We prepared ourselves for the first large clinical trials in FSHD in The Netherlands which will commence in 2022.
Myasthenic syndromes
LUMC is the national referral center for Myasthenic syndromes in the Netherlands. We govern the Dutch-Belgian myasthenia registry, used to collect data on natural history, disease presentation. risk factors, disease severity and treatment effects among other items of interest to get a better understanding of disease variability, and for trial readiness. Fatigue is a common problem in MG for which many factors could be responsible. To get a better understanding of these underlying mechanisms a study started to seek for biomarkers of fatigue in blood and muscle. To advance the diagnostics of MG a facial recognition study is ongoing using artificial intelligence. MRI studies of eye muscle are used to investigate whether visualization of eye muscles could contribute in the diagnosis of MG and could predict the effect of treatment. Alongside multiple pharma-initiated clinical trials are being conducted at the LUMC.
Inclusion body myositis
The LUMC together with Amsterdam UMC and Radboudumc are IBM expert centres. In close collaboration with the department of Human Genetics and partners from the national IBM expert centres, a muscle on a chip model of IBM is being developed. We started to test the role of cytosolic 5’-nucleotidase (anti-Cn-1A) in the pathophysiology of IBM but aim to create a disease model which could be helpful in understanding the disease and which could serve to test drugs in a preclinical phase. Clinical trials to test the efficacy and safety of for example Sirolimus will be implemented. A national registry is being developed.
&width=2475)
&width=1500)