Jongbloed Group - Morphology and Development of Congenital Heart Disease
The first pilar, Basic science. Aimed at mechanisms and sequelae of congenital/structural heart disease, in particular arrhythmias. Special emphasis is on cardiac autonomic innervation, as this drives the conduction system and its role in arrhythmogenesis is increasingly recognised. Arrhythmias related to pathological cardiac innervation patterns due to disturbed epicardial signalling are currently the groups’ main focus. The group uses in vivo and vivo animal models as well as human tissues.
…The first pilar, Basic science. Aimed at mechanisms and sequelae of congenital/structural heart disease, in particular arrhythmias. Special emphasis is on cardiac autonomic innervation, as this drives the conduction system and its role in arrhythmogenesis is increasingly recognised. Arrhythmias related to pathological cardiac innervation patterns due to disturbed epicardial signalling are currently the groups’ main focus. The group uses in vivo and vivo animal models as well as human tissues.
The second pilar, Clinical and anatomical science. aimed at phenotyping the morphological spectrum of congenital heart disease as related to disease outcome, such as arrhythmias. These studies take advantage of the unique Leiden Collection of Congenital Malformations (tpart of the Biobank Congenital Heart Disease) at the dept. of Anatomy & Embryology, as well as of data of clinical care paths (dept. of Cardiology, Heart Lung Center). A special project is the development of dedicated 3D printing models of congenital heart diseases.
The Aim
Background of the clinical problem
Congenital heart disease (CHD) is the most common congenital disease with an incidence of 1:1000 life born children. In the past years, therapeutics options for the treatment of CHD have significantly improved, thus allowing progressively more patients to reach the adult age. Although early survival rates have improved, a major concern remains the occurrence of late complications, including fatal arrhythmias leading to sudden cardiac death (SCD) in subgroups of patients with CHD. Especially the subgroup of patients with right ventricular dysfunction and the Fontan circulation are at risk. Knowledge on cardiac morphology and variations in phenotype is mandatory to understand and predict some forms of complications. In addition, understanding the molecular mechanisms leading to structural changes in cardiac tissues, is essential to eventually being able to interact with disease cascades induced by hemodynamic overload or ischemia in these patients.
…Background of the clinical problem
Congenital heart disease (CHD) is the most common congenital disease with an incidence of 1:1000 life born children. In the past years, therapeutics options for the treatment of CHD have significantly improved, thus allowing progressively more patients to reach the adult age. Although early survival rates have improved, a major concern remains the occurrence of late complications, including fatal arrhythmias leading to sudden cardiac death (SCD) in subgroups of patients with CHD. Especially the subgroup of patients with right ventricular dysfunction and the Fontan circulation are at risk. Knowledge on cardiac morphology and variations in phenotype is mandatory to understand and predict some forms of complications. In addition, understanding the molecular mechanisms leading to structural changes in cardiac tissues, is essential to eventually being able to interact with disease cascades induced by hemodynamic overload or ischemia in these patients.
Of the acquired structural heart diseases, myocardial infarction (MI) is a major cause of death in the developed world. In patients with myocardial infarction, SCD has been related to pathological innervation patterns of the left ventricle, leading to autonomic hyperinnervation with electrophysiological disarray potentially causing electrical instability and SCD. Accumulating evidence suggests a relationship between ventricular arrhythmias, sudden cardiac death and activity of the sympathetic autonomic nerve system.
Aims
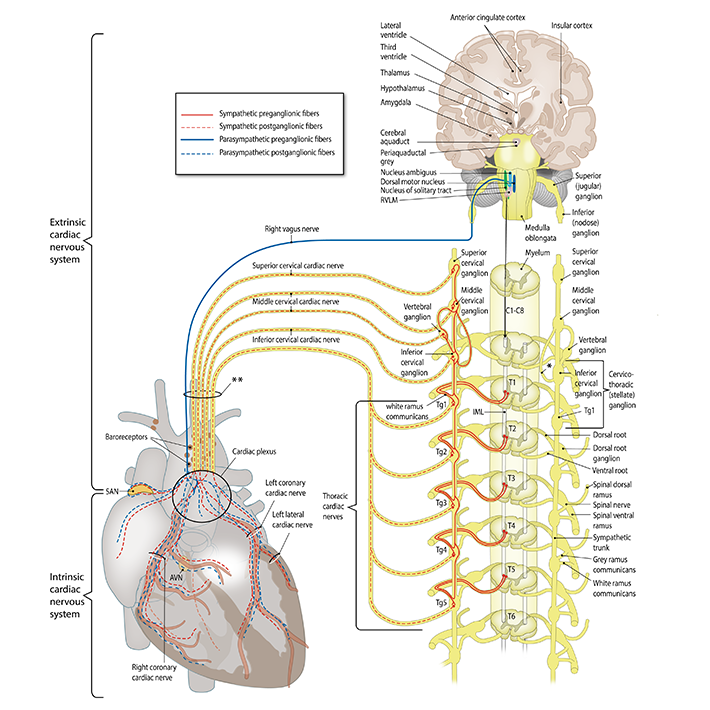
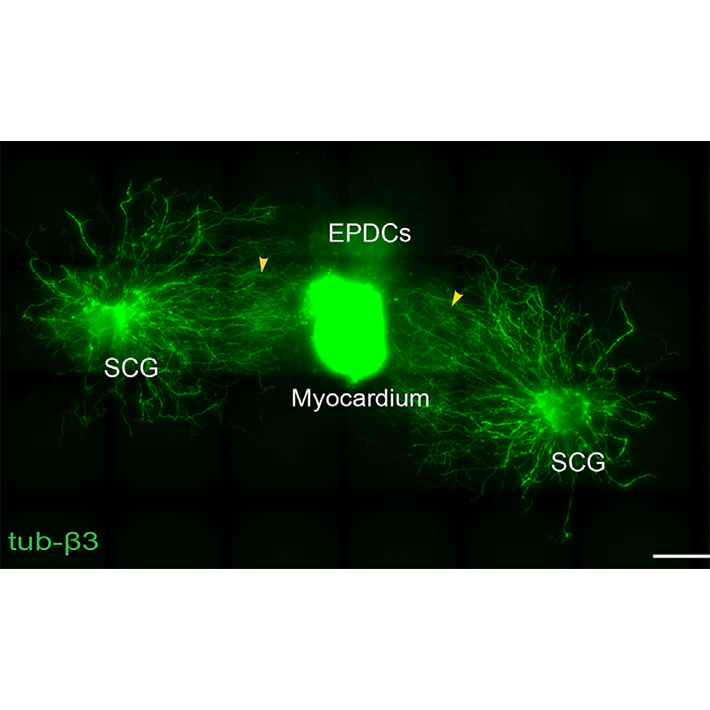
Basic science. Main focus of basic science is aimed on sequalae of congenital and structural heart disease, specifically related to pathomorphology of the cardiac autonomic nervous system (Figure 1) including mechanisms of remodelling of the cardiac autonomic nervous system in structural (acquired and congenital) heart disease. The research group aims to determine the role of different cell types influencing cardiac nervous system development, characterise factors influencing neuronal outgrowth and identify prerequisites for cardiac (re)innervation (Figure 2). This includes analysis of neurotrophic factors expressed/secreted by cardiac cells and genetic characterization of pathways involved in neuronal remodelling after cardiac damage by snRNAseq. Sequelae of cardiac damage in terms of arrhythmias related to pathological innervation patterns, are studied in an (transgenic) animal models as well as in human tissues.
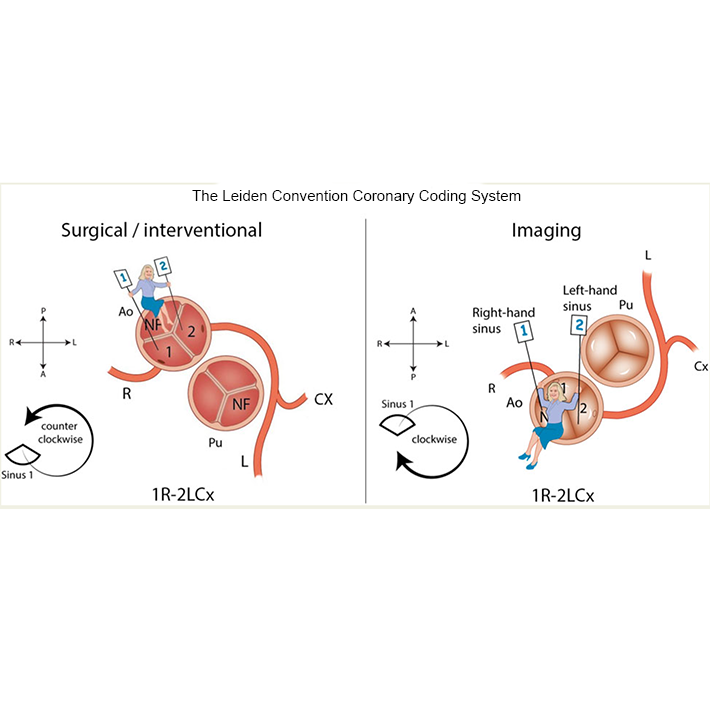
Clinical Research. The aim of our clinical research is to explore cardiac morphology as related to determinants of clinical outcome in different forms of congenital heart disease. Morphological variations in phenotype have in the past years been explored in different forms of congenital heart disease, including AVSD, tetralogy of Fallot, AVSD, bicuspid aortic valve, TGA and anomalous coronary arteries (Figure 3).
The group leader has a dual appointment at the deps. of Anatomy & Embryology and Cardiology of the LUMC, and is a member of the clinical adult congenital heart disease (ACHD) group of the Center for congenital heart disease Amsterdam-Leiden (CAHAL).
Clinical research, performed in collaboration with the other members of the clinical LUMC ACHD team, currently focusses on pathomorphological aspects and treatment strategies in adult patients with a failing (systemic) right ventricle, the Fontan circulation and on anomalous coronary arteries. The ACHD group also has a leading role in the MuSCAT trial (Multicenter Study on Coronary Anomalies in The Netherlands). The substudy ”MusCat Histology” is performed in a collaboration with the dept. of Anatomy & Embryology. The LUMC is a national referral center for advanced systemic right ventricular heart failure and ventricular assist device implantation as ‘destination therapy’ in this patient group.
Head of the group
Dr. Monique Jongbloed, cardiologist, associate professor is the head of this research group. Read more at https://orcid.org/0000-0002-9132-0418.
Clinical anatomy-3D printing of congenital malformations
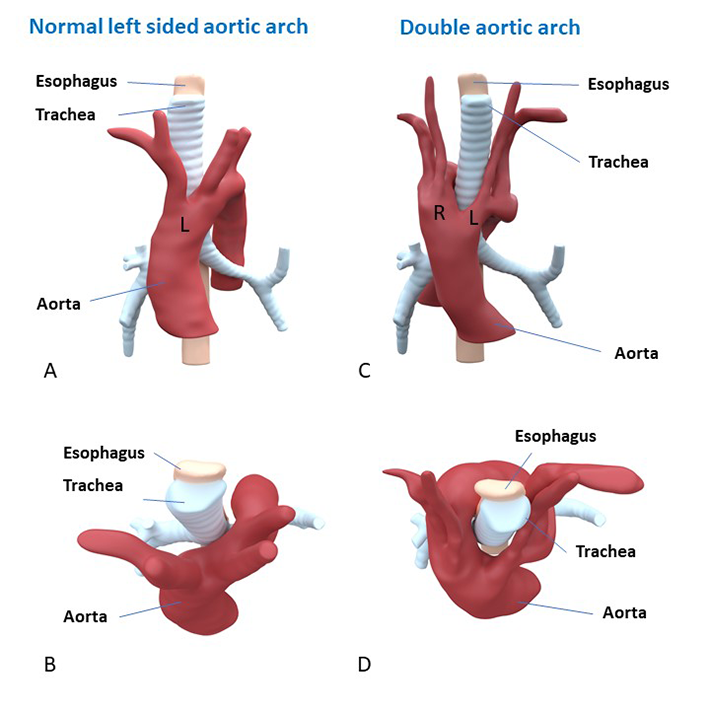
A special project is the development of dedicated 3D printing models of congenital heart diseases for educational and procedural planning purposes, that are developed on the dept. of Anatomy & Embryology (Figure 4). The complex anatomy, treatments and risk of late complications may have an important impact on the life of congenital heart disease patients. Adequate knowledge transfer is very important for disease insight and understanding, not only for the patient, but also for his/her family. This project is aimed at improving information for patients with congenital heart defects and their family members through 3D printing. We have developed protocols for creation of detailed colour 3D prints of congenital heart defects, based on CT and MRI scan of patients. In the past years, several 3D printing models of congenital heart defects have been developed, that are used in the consultation room for patient education.
…A special project is the development of dedicated 3D printing models of congenital heart diseases for educational and procedural planning purposes, that are developed on the dept. of Anatomy & Embryology (Figure 4). The complex anatomy, treatments and risk of late complications may have an important impact on the life of congenital heart disease patients. Adequate knowledge transfer is very important for disease insight and understanding, not only for the patient, but also for his/her family. This project is aimed at improving information for patients with congenital heart defects and their family members through 3D printing. We have developed protocols for creation of detailed colour 3D prints of congenital heart defects, based on CT and MRI scan of patients. In the past years, several 3D printing models of congenital heart defects have been developed, that are used in the consultation room for patient education.
Several of these models can be found at: https://anatomytool.org/content/congenital-heart-diseases
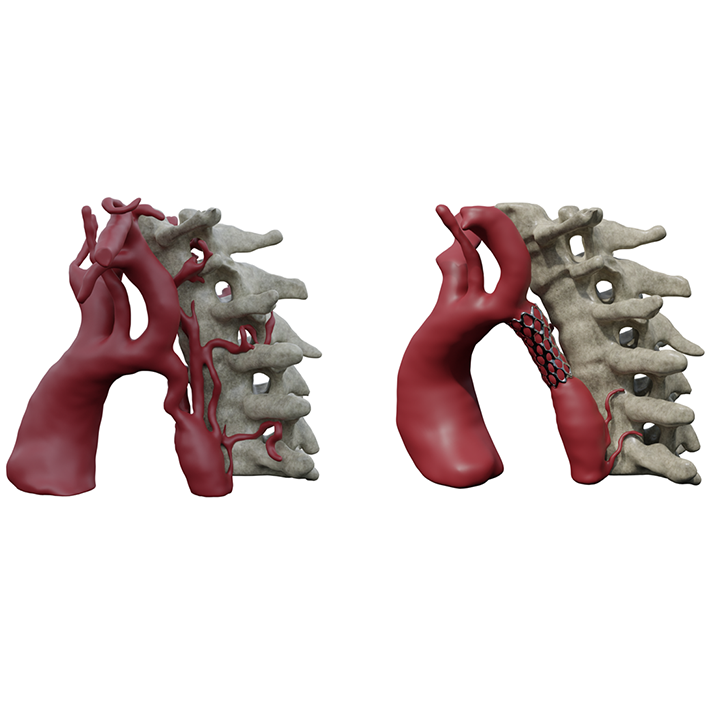
In addition, personalised print are used for procedural planning, such the treatment of aortic coarctation by stenting (Figure 5).
The project is part of the activities of the Expertise Center for Cardiovascular Development and Disease (ECCARD).
Themes for innovation and Societal Outreach
Key publications
Our team
Group members
- Monique R.M. Jongbloed (Cardiologist, Associate Professor)
- Conny van Munsteren (Research Technician)
- Bert Wisse (Research Technician)
- Margot Bartelings (Assistant professor)
Collaboration with:
Marco C. de Ruiter (PI, Professor)
PhD students
- Michiel Blok (MsC)
- Sophia Chen (MD)
- Leo Engele (MD)
- Claire Glashan (MD)
- Claire Koppel (MD)
- Ruben Methorst (MsC)
- Marieke Nederend (MD)
- Liza Polyokova (MsC)
- Lieke van Roon (BsC)
- Tjitske Zandstra (MD)
- Fleur Zwanenburg (MD)
&width=710)
&width=710)
&width=710)
&width=710)
&width=710)