Interventional/intraoperative Molecular Imaging
Building on the expertise available in our highly multidisciplinary team, we continuously try to generate innovations that advance minimally invasive interventions. We actively strive to translate technologies to the clinic and make the impossible possible.
Chemical innovations in image-guided therapy
Critical for molecular imaging applications is the ability to highlight specific biological processes or biomarkers. Since endogenous contrast is notoriously heterogeneous in its expression, as a group we develop disease- or anatomy specific exogenous contrast agents. In the field of molecular imaging, these agents are referred to as ‘tracers’ and can have for example a radioactive, fluorescent, mass-spectrometry or magnetic signature. Since each individual signature has advantages and disadvantages, many of our chemical efforts revolve around synthesizing bi-modal or hybrid tracers that hold complementary imaging signatures e.g., fluorescence and radioactivity. In close collaboration with the LUMC pharmacy and GMP radiochemistry facility we set up GMP-production protocols and produce clinical grade tracers for in human evaluation, so-called academic pharma.
Tracers developed within our chemistry labs are biologically evaluated in vitro, in vivo, and ex vivo. During such evaluations we make use of advanced disease models and top-notch preclinical evaluation settings to carefully document affinity, specificity, pharmacokinetics, and toxicity. One critical aspect during these evaluations is always the molecular structure activity relation and the technologies’ ability to address the needs of clinical end-users.
Focus area’s:
- Tumor targeting (main end-users: nuclear medicine, interventional radiology, urology, general surgery, head and neck surgery, and orthopedic surgery)
- Nerve sparing surgery (main end-users: all surgical disciplines)
- Bacterial infections (main end-users: orthopedic surgery, infectious diseases, microbiology)
- Vaccine design (main end-users: infectious diseases, parasitology)
Medical devices for image-guided surgery
Advanced imaging tracers have limited clinical value when they are not complemented with high-end imaging modalities, so-called enabling technologies. While in some cases we adapt the tracers to existing modalities, in most cases tracer implementation creates a need for new medical devices. Via a strong collaboration with the LUMC department of Medical Technology (a.o. Design and prototyping) and the Leidse instrument makers school (LiS) we produce new surgical instruments and modalities in-house. Following CAD-design and 3D printing we generate fully functional prototypes suitable for preclinical evaluation. When these initial steps prove succesfull, we creat
e prototypes that are clinical compatible (MDR compliant) and train our clinical partners in their use. The resulting medical devices provide the basis for clinical valorization trials.

Our engineering efforts directly align with our chemical efforts, meaning we design modalities specific for (intraoperative) fluorescence, radioactivity and magnetic detection, or combinations thereof. Hardware components are complemented with matching software algorithms (e.g. automated video analysis) that we develop.
Focus areas:
- Fluorescence-guided surgery (key devices: (multicolor)fluorescence camera’s, smart instruments)
- Radioguided surgery (key devices: steerable detectors, hybrid modalities)
- Surgical navigation (key devices: AR/VR displays, freehand scanners)
- Performance evaluation (key devices: trackable instruments, neural networks for instrument tracking and kinematic analysis algorithms)
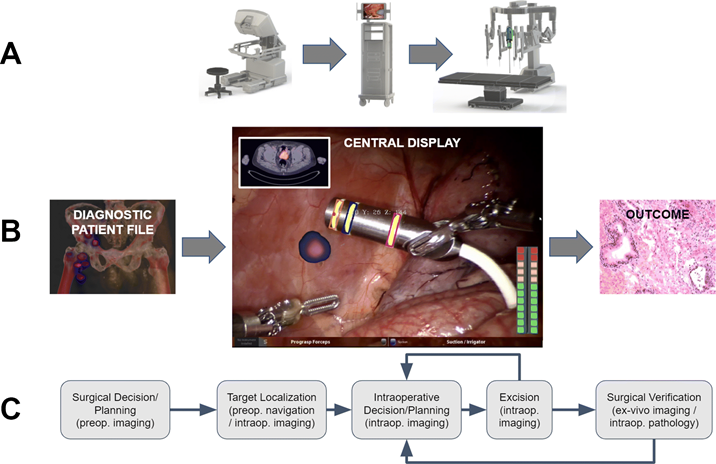
(Clinical) valorization
The underlying aim of creating new medical technologies (both chemical- and medical device-based) is to advance the level of care for the patient and/or facilitate the medical professional. To allow this, as a group, we spend a substantial portion of our time translating new innovations from the lab or workshop into clinical valorization studies. We pursue such translations in the LUMC and in other key centers of expertise e.g. NKI-AvL and Martini Klinic. Currently most of our clinical efforts are focused on oncologic surgery. Based on in-house technology design (see other praragraphs) we can pursue investigator driven phase 1 feasibility studies. If these are successful, we follow up with phase 2 validation trails (preferably randomized and multicenter). It is important to note that moving beyond first-in-human translation helps us to determine how (our) technologies impact on clinicalparameters such as outcome and complications. Hence, we follow up on our most promising technologies and apply them in large patient groups were possible e.g., the hybrid tracer ICG-99mTc-nanocolloid has been applied in > 2000 patients. The large cohort studies are used to guide widespread clinical implementation.
To ensure valorization, and help facilitated adoption in routine care, we align the needs of medical end-users with commercial partners that can bring ‘our’ technologies to market. Examples of commercial products that we (co-) developed: ICG-99mTc-nanocolloid (GE Healthcare), Opto-nuclear probe (Eurorad), DROP-IN gamma probe (Crystal Photonics GmbH), Image1 S Laparoscope (Karl Storz), PDE-neo II (Hamamatsu Photonics).
To increase visibility of our technologies for end-users, industry, and patients, we actively pursue public engagement activities in the form of medical training, hands-on demonstrations and public education.
Fluorescence-guided sentinel node excision
&width=826&height=464)