Cellular Immunometabolism Group
Immunemetabolism of myeloid cells
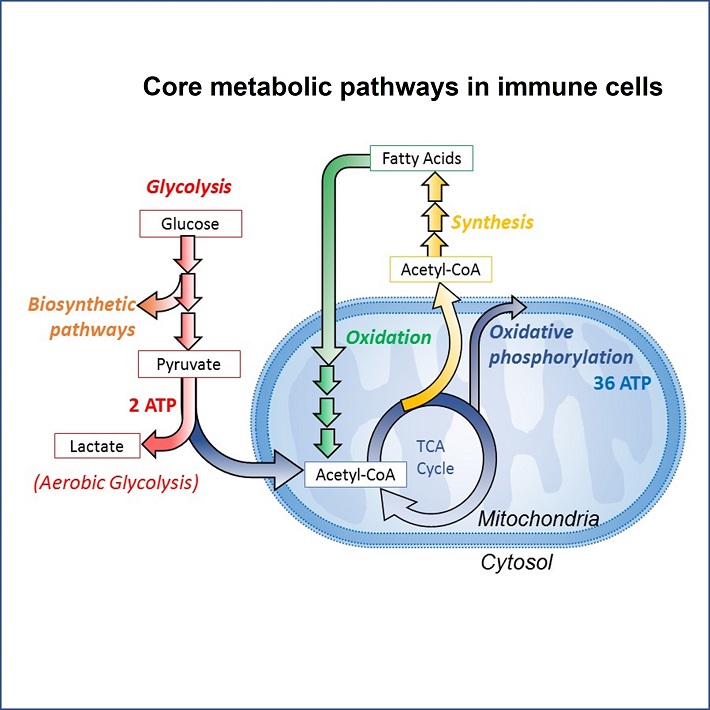
Dendritic cells (DCs) and macrophages are both myeloid cells with central roles in the initiation and regulation of immune responses in the context of infections as well as inflammatory disorders and cancer. DCs have superior antigen presenting capacities and are crucial for the initiation and polarization of T cell responses during an immune response. Macrophages are well known for their ability to remove and phagocytose cellular debris and microbes and, when polarized towards an anti-inflammatory state, to mediate tissue repair and homeostasis. There is significant interest in understanding how their function is regulated and what kinds of stimuli are needed to initiate/sustain their activation and polarisation in specific scenarios. There has been a longstanding interest in defining the signalling pathways regulating macrophage and DC function in the context of “classical” immunological cues, such as cytokines, chemokines, pathogen-associated molecular pattern (PAMPs). More recently, there has been a growing appreciation that also metabolic signals and alterations in cellular metabolism can dictate immune cell function. This field of immunometabolism has contributed to the realization that stimuli and changes in the environment macrophages and DCs are exposed to, eventually converge into alterations in their metabolic properties. It has become clear that reprogramming of metabolic pathways such as glycolysis, oxidative phosphorylation, fatty acid synthesis and oxidation are not only associated with, but are also crucial for shaping functional responses of DCs and polarization of macrophages to environmental cues.
…Immunemetabolism of myeloid cells
Dendritic cells (DCs) and macrophages are both myeloid cells with central roles in the initiation and regulation of immune responses in the context of infections as well as inflammatory disorders and cancer. DCs have superior antigen presenting capacities and are crucial for the initiation and polarization of T cell responses during an immune response. Macrophages are well known for their ability to remove and phagocytose cellular debris and microbes and, when polarized towards an anti-inflammatory state, to mediate tissue repair and homeostasis. There is significant interest in understanding how their function is regulated and what kinds of stimuli are needed to initiate/sustain their activation and polarisation in specific scenarios. There has been a longstanding interest in defining the signalling pathways regulating macrophage and DC function in the context of “classical” immunological cues, such as cytokines, chemokines, pathogen-associated molecular pattern (PAMPs). More recently, there has been a growing appreciation that also metabolic signals and alterations in cellular metabolism can dictate immune cell function. This field of immunometabolism has contributed to the realization that stimuli and changes in the environment macrophages and DCs are exposed to, eventually converge into alterations in their metabolic properties. It has become clear that reprogramming of metabolic pathways such as glycolysis, oxidative phosphorylation, fatty acid synthesis and oxidation are not only associated with, but are also crucial for shaping functional responses of DCs and polarization of macrophages to environmental cues.
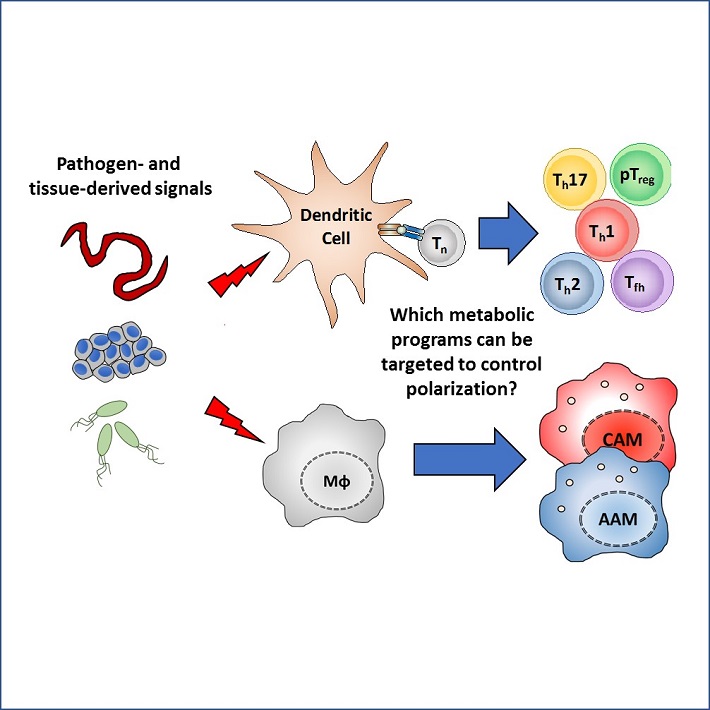
Our group aims to unravel the metabolic cues present in the microenvironments and intracellular metabolic programs that shape the T cell-polarizing properties of DCs and the polarization state of macrophages in infection and cancer. Ultimately, the goal is to identify novel metabolic pathways in DCs and macrophages that could be targeted to direct their function for improving vaccination efficacy and cancer therapies.
Main research questions:
- What are the metabolic requirements for DCs to prime and polarize different T cell responses? (project 1,2,3)
- How does the metabolic micro-environment affect the functional properties of dendritic cells and macrophages in cancer, obesity and autoimmune diseases? (Project1, 2, 4)
- How do metabolic sensors AMPK and mTOR shape the T cell priming and polarizing properties of dendritic cells? (project 1)
- How do dendritic cells prime Th2 responses in response to parasitic helminths? (Project 3)
- Can immune cell metabolism be targeted to reverse immune- and vaccinehyporesponsiveness? (project 5)
Methodology and Tools:
- Metabolic flux analysis
- High dimensional flow cytometry
- Untargeted metabolomics
- RNA sequencing
- In vitro culture systems of human DCs and macrophages
- Murine models of infection and cancer with conditional KO mice
&w=710&h=710)
&w=710&h=710)
Projects
Themes for innovation / Societal Outreach
Publications
Our Team members
Dr. Bart Everts (PI)
Eline Brombacher (PhD student; project 1)
Eunice Betouke Ongwe (PhD student; project 5)
Marjolein Quik (PhD student; project 2)
Luis Almeida (PhD student; project 3,4)
Dr. Joana Bras Nunez (Postdoctoral fellow; project 1)
Dr. Graham Heieis (Postdoctoral fellow; project 2,5)
Dr. Thiago Patente (Postdoctoral fellow; project 1)
Ing. Alwin van der Ham (Technician)
Frank Otto (Technician)